What Is the Hybridization State of Si in H3si Sih3
A True B False 70. B trigonal bipyramidal t-shaped.

In Ch3 3n Nitrogen Is Sp 3 Hybridized Whereas In Sih3 3n It Is Sp 2 Hybridised Why
So the structure is given as-.

. What is the hybridization state of Si in SiH4 and in H3Si-SiH3. The hybridization of Si in SiO2 is sp3Si. S i has vacant d orbital which can take part in back bonding ie P Π d Π back bonding by accepting back the lone pair of electrons of O atoms to its vacant d orbital.
Starting with Lewis structure determine the number and type of hybrid orbitals necessary to rationalize the bonding in SiH 4. A SiBr4 and bBlC3. Consider the reaction BF 3 NH 3 F 3 B NH 3.
The Lewis structure of SiH 4 is. Hybridization In valence bond theory for an atom to form a covalent bond with another it must create new hybrid orbitals by mixing sp and d orbitals. It valance orbital diagram is.
Hybridization is the concept of mixing of similar shape and energetic atomic orbitals that form new hybrid orbitals with different energies shapes than the atomic orbitals. The S i hybrid h S i is of s p X 316 character as expected. What is the hybridization state of SI in SIH4.
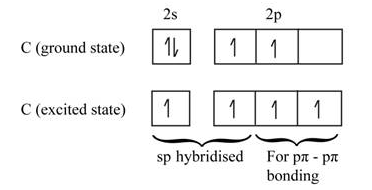
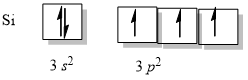
The ground state electron configuration of the Si atom is Ne 3 s2 3 p2. T his is also showung tetravalent state its hybridized state configuration will be 1s2 2s2 2p6 3s1 3px1 3py1 3pz1 as one e- is promoted from 4s to 3pz to produce 4 unpaired e available for making bonds. This results in a pi bond between the d and p orbitals which is called a d π -p π bond.
Si can also be found in sp3 hybrid state so. On putting these values we get-. What is the hybridization state of Si in SiH4 and in H3SiSiH3.
C Si and C are in different groups on the periodic table. Up to 256 cash back What is the hybridization of Si in SiH. Ne3s23p2SiO2 forms a covalent network in.
Step 1 of 5. A Si has a d orbital to which water can add. Enter the numbers corresponding to the correct geometry in the boxes next to the formula.
Step 1 of 4. What is the hybridization state of Si in SiH4 and in H3Si-SiH3. 100 6 ratings for this solution.
Loose Leaf Version for Chemistry. In agreement with Jans answer. B SiCl4 is polar and CCl4 is not.
Steric number of an atom 4 0 4. 7th Edition Edit edition Solutions for Chapter 10 Problem 32QP. Students also viewed these Organic Chemistry questions Give the hybridization state of each carbon in the following Compounds.
Here the number of hydrogen atom bonded with silicon 4 and number of lone pair left on silicon 0. For unlimited access to Homework Help a Homework subscription is required. Draw the structure of 1 IF7 2 H3O Calculate their hybridization and also determine the shapes and geometries arrow_forward Determine the hybridization state of the underlined atoms in the following compounds.
The hybridization of S i H 3 2 O has been changed from s p 3 to s p 2. Chemistry questions and answers. D The Si-H bond is shorter than the C-H bond.
In the hydrocarbon a What is the hybridization at each carbon atom b How many Ï bonds are there in the molecule. 100 39 ratings for this solution. The N atoms in the molecule below are in two hybridization states.
D trigonal planar trigonal planar. Electron Domain Geometry Molecular Geometry a NH4. The N hybrid h N is of s p X 200 character.
Now we know that if the steric number is 4 then hybridization is s p 3 and geometry is tetrahedral. Predict the geometries of the following ions. Hybridisation and shape of N in H3Si3N.
The shape of trisilylamine H 3 Si 3 N is planar this is due to the fact that one of the empty d orbitals in Silicon interacts with the p orbital of Nitrogen containing the lone pair of electrons. What is the hybridization state of Si in SiH4 and in H3Si-SiH3. What is the hybridization of silica in silicon dioxide.
The molecule B2 is diamagnetic. The N S i bond is polarised towards N 09000 h N 04359 h S i as expected due to electronegativity. The hybridization state of Si in SiH4 is sp3 hybridized and in H3Si SiH3 it is also sp3 hybridized 1034.

Welcome To Chem Zipper Com 2020

Explain The Change In The Hybridization Of Sih3 2 O
Answered What Is The Hybridization Of The Bartleby

The Hybridization State Of Nitrogen Atoms In The Molecules Sih 3 3 N And Ch 3 3 N Are

Answered What Is The Hybridization State Of Si Bartleby

Data Driven Investigation Of Monosilane And Ammonia Co Pyrolysis To Silicon Nitride Based Ceramic Nanomaterials Choi 2020 Chemphyschem Wiley Online Library
Solved What Is The Hybridization State Of Si A In Sih4 And B Chegg Com

Welcome To Chem Zipper Com 2020

Solved Be Sure To Answer All Parts What Is The Chegg Com

Q Why N Ch3 3 Is Pyramidal But N Sih3 3 Is Planar While Both C And Si Belonging To Same Group Youtube
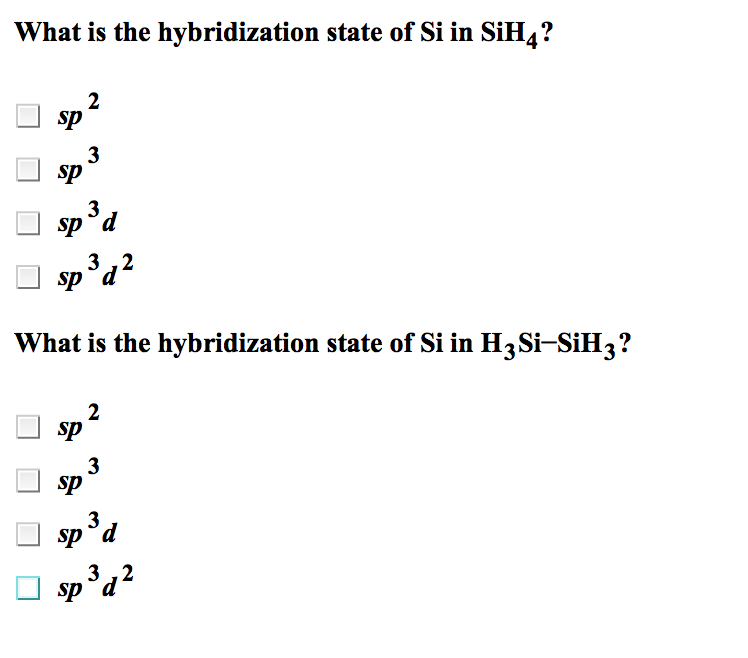
Solved What Is The Hybridization State Of Si In Sih 4 Sp 2 Chegg Com
What Is The Hybridization State Of Si A In Sih4 And B Studysoup
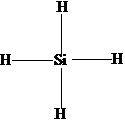
Solved What Is The Hybridization State Of Si A In Sih4 And B Chegg Com
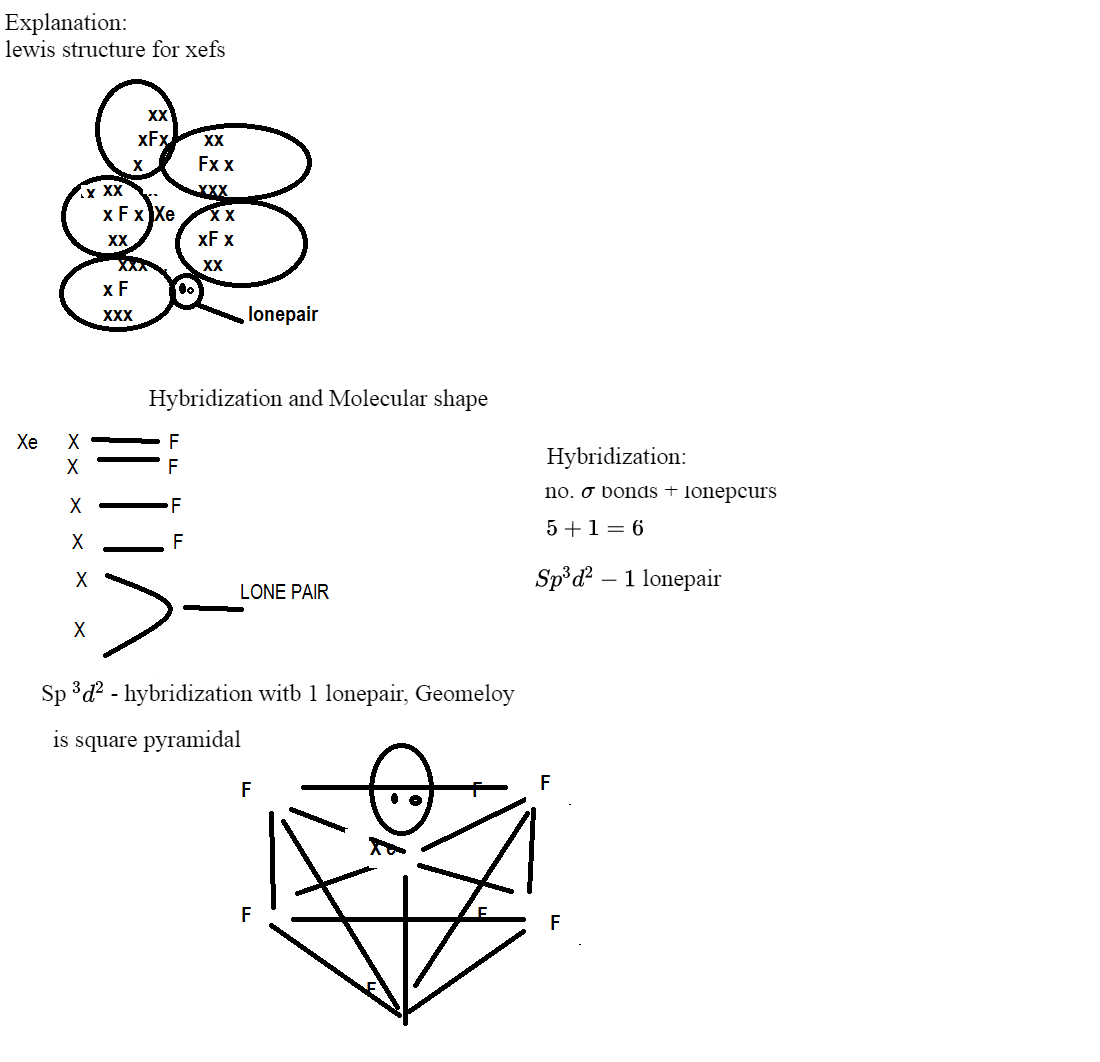
Answered Draw The Lewis Structure For Xef5 And Bartleby

Answered What Is The Hybridization State Of Si Bartleby

Hybridization Structure Of Trisilyl Amine N Sih3 3 And Trimethyl Amine N Ch3 3 Basic Strength Youtube

Solved Describe The Bonding Scheme Of The Ash3 Molecule In Chegg Com


Comments
Post a Comment